CH2O C2Cl4 CH3NH2 CFCl3 C. Draw a Lewis structure for each of the following molecules.
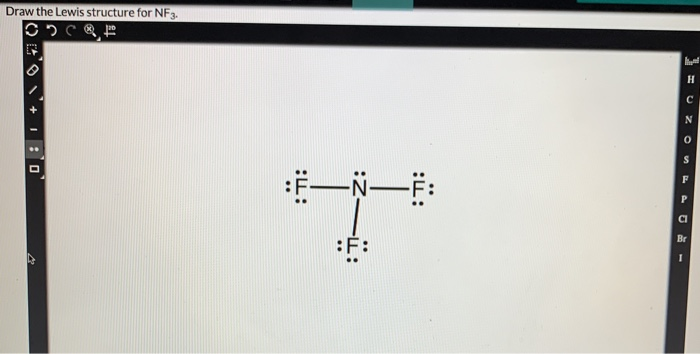
Solved Draw The Lewis Structure For Nf3 Part 2 1 Point Chegg Com
Contains one N and three I atoms d.

. CH2O Carbon is the central atom 4. Linear trigonal planar tetrahedral as well as the. A Draw the Lewis structure for NF 3.
NF3 Nitrogen is the central atom 2. What are its electron-pair geometry and molecular geometries. First week only 499.
Draw a Lewis structure for each of the following molecules. Include all lone pairs of electrons. Steps of drawing lewis structure of NF 3 Total number of electrons of the valance shells of NF 3.
Include all lone pairs of electrons and nonbonding electrons. Draw the Lewis structure for NF3. There are two elements in NF 3.
See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. There are two elements in NF 3. CH3Cl Carbon is the central atom 3.
Draw Lewis structures for each of the following molecules. Nitrogen in the NF3 Lewis structure with all three fluorine atoms arranged in a trigonal pyramidal geometry. BF3 CO2 NF3 NO2.
A lewis structure is a diagram that shows the bonding between atoms of a molecule. Solution for Draw a Lewis structure for each of the following molecules. What are its electron-pair and molecular geometries.
A NF3 b ClO3 c HOBr d SO32. What is the. What is the hybridization of the n LIMITED TIME OFFER.
Add valence electrons around the fluorine atom as given in the figure. Start your trial now. What is the hybridization of the N atom.
Write a Lewis structure for each molecule. Draw the Lewis structure for NF3. Up to 256 cash back Get the detailed answer.
Total valence electrons pairs. Draw a valid Lewis dot structure for the following molecules a NCOb NF3 12 from CHEM 100 at Joliet Junior College. Draw the Lewis structure for NF 3.
Contains one C and four F atoms b. Nitrogen has five valence electrons while fluorine has seven. Up to 256 cash back Get the detailed answer.
Lets move forward to the steps of drawing the Lewis structure of NF3. Lewis structure of NF3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. What are its electron-pair and molecular geometries.
Include all lone pairs of electrons and nonbonding electrons. Draw your final diagram as a 3-dimensional structure. Contains one Si and four Br atoms e.
NF3 HBr SBr2 CCl4. See answer 1 Best Answer. Connect the exterior and core central atom of the NF3 molecule with three single N-F bonds.
Since Hydrogen is in Group I it has one 1 valence electron in its shell. Draw a Lewis structure for each of the following molecules. Lewis dot Structure for NF3 generated from step-1 and step-2.
POCl3 SO42 XeO4 PO43 ClO4 b. Include all lone pairs of electrons and nonbonding electrons. Contains two H and one Se atom c.
In the modern periodic table Fluorine being a halogen belongs to group 17 and nitrogen to group 15. A step-by-step explanation of how to draw the NF3 Lewis Dot Structure Nitrogen trifluorideFor the NF3 structure use the periodic table to find the total n. Draw a Lewis structure for each of the following molecules or ions.
See the answer See the answer done loading. NF3 HBr SBr2 CCl4 Write a Lewis structure for each molecule. H2S SiCl4 BeF2 CO2 - 3 and HCOOH.
Chemistry and Chemical Reactivity Enhanced Review Edition with General ChemistryNOWÃâžÂ 6th Edition Edit edition Solutions for Chapter 9 Problem 11PS. Draw the Lewis structure for NF 3. How to Draw a Lewis Structure for NF3.
What orbitals on N and F overlap to form bonds between. Count the total number of valence electrons present on each atom of the NF3 molecules. NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds and NCl3 lewis structure has 3 chlorine and 1 nitrogen connected with three single bonds also.
Up to 24 cash back For each of the following molecules draw a Lewis structure and predict the molecular geometry hybridization overall polarity and IMFs of the molecule. 8 rows Simple steps for drawing the NF3 lewis dot structure. Contains one C one Cl and three H atoms.
What is the hybridization of the N atom. What is the Lewis structure for NF3. Nitrogen in the NF3 Lewis structure with all three fluorine atoms arranged in a trigonal pyramidal geometry.
Draw Lewis structures for the following molecules count the number of atoms bonded to the central atom and the lone pairs on the central atom and assign the correct electron pair and molecular geometries for each. The following geometries may be used. Include all lone pairs of electrons.
What are its electron-pair geometry and molecular geometries. Draw the Lewis Dot Structure for the Hydrogen atom. Weve got the study and writing resources you need for your assignments.
NF3 1 answer below. Count total valence electron in. Total electron pairs are determined by dividing the number total valence electrons by.
GET 20 OFF GRADE YEARLY SUBSCRIPTION. A step-by-step explanation of how to draw the NF3 Lewis Dot Structure Nitrogen trifluorideFor the NF3 structure use the periodic table to find the total n. This type of Lewis dot structure is represented by an atomic symbol and a series of dots.
CH2O C2Cl4 CH3NH2 CFCl3 C central. Click hereto get an answer to your question Draw the Lewis structures for the following molecules and ions.

Vsepr Megavideo 36 Examples Including Lewis Structure Molecular Geometry Intermolecular Forces Youtube

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
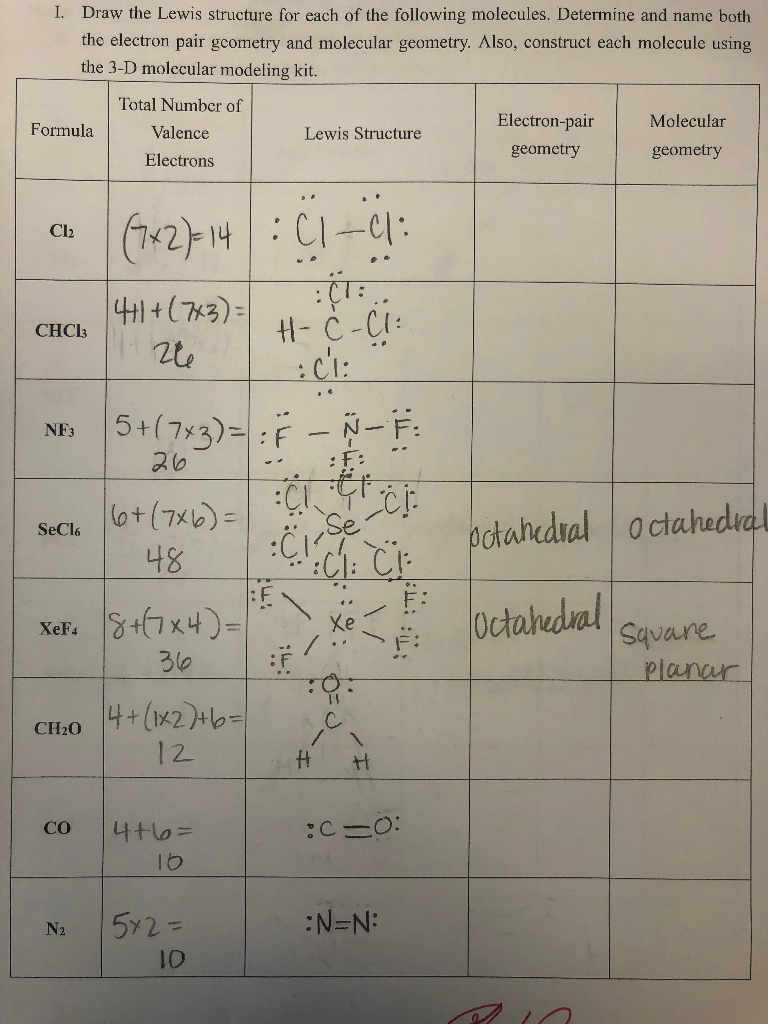
Solved Draw The Lewis Structure For Each Of The Following Chegg Com

Nf3 Lewis Structure Nitrogen Trifluoride Math Molecules Lewis

Draw The Lewis Structures For The Following Molecules And Ions H2s Sicl4 Bef2 Co 2 3 And Hcooh

Nf3 Lewis Structure Nitrogen Trifluoride Youtube
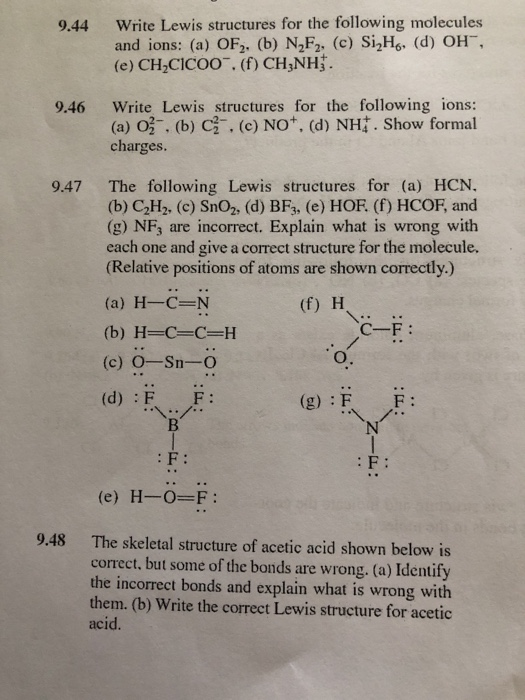
Solved Write Lewis Structures For The Following Molecules Chegg Com
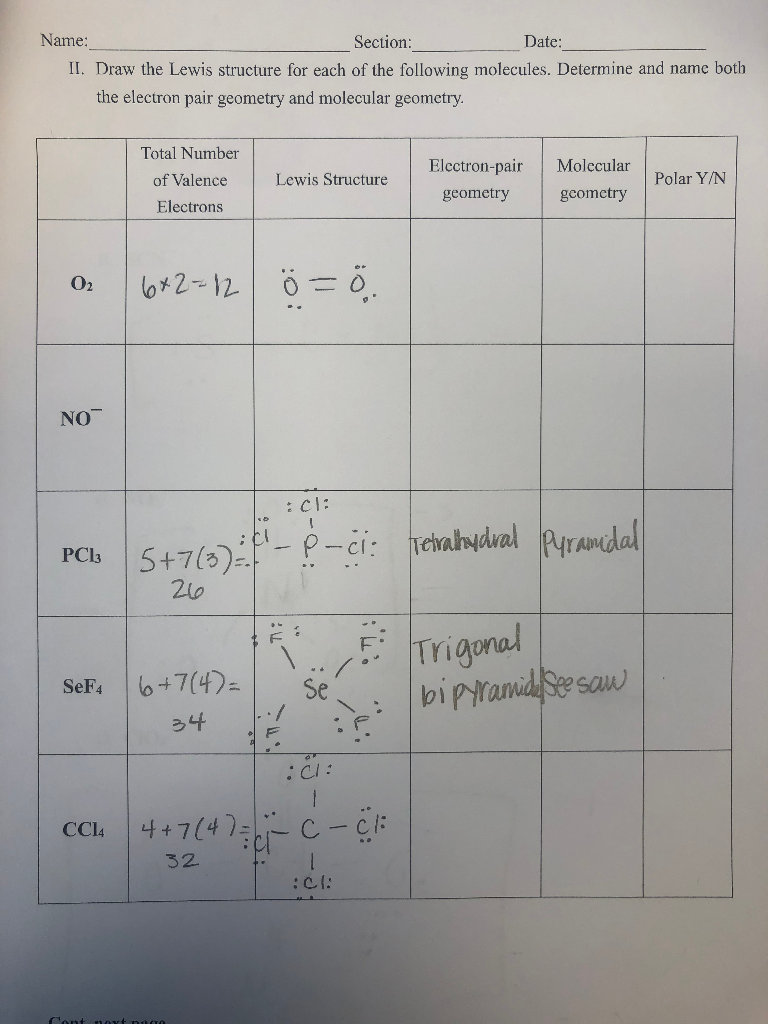
Solved Draw The Lewis Structure For Each Of The Following Chegg Com
0 comments
Post a Comment